
The same argument goes for the adiabatic process you mentioned.

Raising its temperature and doing external work. Addition of heat at constant pressure to a gas results in. Related Questions on Engineering Thermodynamics. So, you see $dU = mC_vdT$ whether or not there is any change in volume. In words, work done in an adiabatic process depends only on the end states (determined by U). So we can see that the heat $dQ_u$ is only used to change the internal energy and not the volume and so $dQ_u = dU$ and we can say according to the definition of $C_v$ the following $$C_v = \frac\ \Rightarrow\ dQ_u = mC_vdT\ \Rightarrow\ dU=mC_vdT$$ So instead of writing $dQ = dU + dW$ we write, $$dQ = dQ_u +dQ_w$$ And the rest of the heat will eventually account for the change in volume, and say that is $dQ_w$. An adiabatic process is can be achieved if the expansion or compression of gas is carried in a perfectly insulated system or is carried out so fast that heat. Now we know some of that heat is going to account for the change in internal energy, let's say that amount is $dQ_u$. Say we applied $dQ$ amount of heat to the a gas. Now let's consider a case where change in volume is allowed. So a different way to define $C_v$ is to say, it is the amount of heat required to raise the temperature of 1 mol of the gas by $1$ K, where the said heat is used to only to change the internal energy U or so to speak the temperature and nothing else. (Note that increasing U essentially means increasing the temperature since U is a function of T for any ideal gas.) Now if we were to keep the volume constant somehow, then the total applied heat would be used only to increase the internal energy or so to speak the temperature and nothing else. When we apply heat to an ideal gas, some of that heat is used to increase the internal energy $U$ of the gas and the rest is eventually used up to do the work to increase its volume.

The molar specific heat at constant volume $C_v$ is basically the amount of heat required to raise the temperature of 1 mol of the gas by $1$ K while keeping the volume constant.
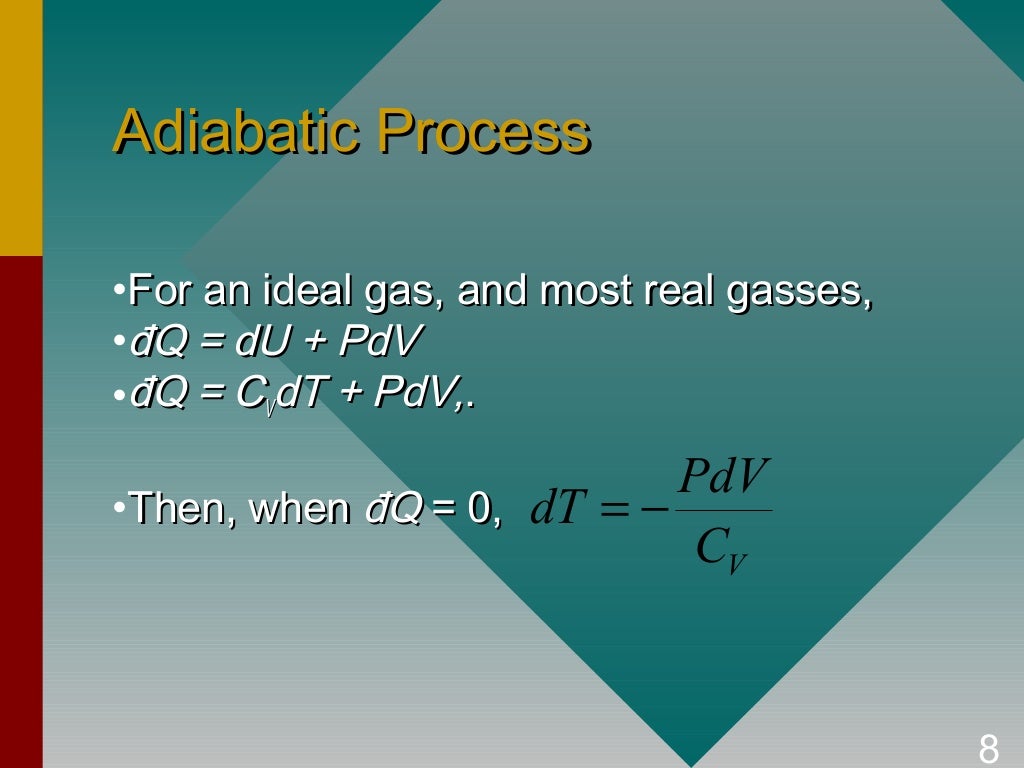
I will just expand on what Chester Miller had already said. 6.09 Work Energy Theorem For a Variable Force. It is just that, in these other situations (involving work), dQ is not equal to $C_vdT$. Learn work done in adiabatic process, derivation and equation, topic helpful for cbse class 11 physics chapter 12 thermodynamics, neet and jee preparation. At constant volume, we can determine the heat capacity of the material by measuring the heat transferred $dQ=dU=C_vdT$.īut this same heat capacity also applies to all other situations for an ideal gas if we recognize that, for an ideal gas, $U$ depends only on temperature, such that $dU=C_vdT$. Even though we call $C_v$ the heat capacity at constant volume, what we really mean by the subscript v is that this is the way we measure $C_v$.


 0 kommentar(er)
0 kommentar(er)
